The compositions in the (100 − x)La0.6Sr0.4CoO3±δ-xCeO2 (LSCC) system with 5 ≤ x ≤ 76 are two-phase at room temperature. They consist of the modified perovskite with rhombohedral symmetry (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c) and modified ceria with fluorite structure (Fm
c) and modified ceria with fluorite structure (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m). The cross-dissolution of La, Sr, Co and Ce cations between the initial La0.6Sr0.4CoO3±δ (LSC) and CeO2 takes place and results in the modification of the initial phases. This is particularly important for the modified ceria. The lattice parameter of the modified ceria increases due to the dissolution of La and Sr cations with larger ionic radii, thereby changing noticeably the oxygen sublattice in the fluorite structure. Above 300 °C LSCCx composites are three-phase due to the reversible change in the symmetry from rhombohedral (R
m). The cross-dissolution of La, Sr, Co and Ce cations between the initial La0.6Sr0.4CoO3±δ (LSC) and CeO2 takes place and results in the modification of the initial phases. This is particularly important for the modified ceria. The lattice parameter of the modified ceria increases due to the dissolution of La and Sr cations with larger ionic radii, thereby changing noticeably the oxygen sublattice in the fluorite structure. Above 300 °C LSCCx composites are three-phase due to the reversible change in the symmetry from rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c) to cubic (Pm
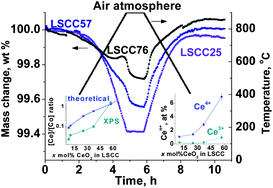
c) to cubic (Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) within the perovskite phase. Red-ox behaviour of the LSCC composites has been explored under air and argon atmospheres in terms of evolution of the chemical composition at the grain's surface and phase interfaces, formation of oxygen vacancies and thermochemistry of this process. Reversible red-ox behaviour was observed in LSCCx with x = 8–37 most probably due to an observed high surface concentration of Co cations, that can be easily involved in the reduction/re-oxidation cycle. The increase in the surface concentration of Ce4+ cations together with the decrease in surface concentration of Co cations seems to result in the differences in the reduction and oxidation behaviour under air in LSCCx with x = 57–76. Formation of oxygen vacancies in LSC, LSCC02 and LSCCx with x = 5–76 in air was not accompanied by any distinct thermal events. This process becomes more endothermic with further increase in oxygen nonstoichiometry (δ) above certain values: δ > 0.08 in LSC, δ > 0.13 in LSCC02, and LSCC with x = 5–76. The LSCCx with x = 5–37 and with x = 57–76 show slightly different reduction behaviour under a(o2) = 7.4 × 10−5. In the composites with a relatively low CeO2 content, the extent of the reduction is proportional to the Co content in a composition, whereas the reduction of the LSCCx with x = 57–76 was more significant than expected. The changes in the enthalpy of oxygen vacancy formation and the kinetics of reduction have been discussed.
m) within the perovskite phase. Red-ox behaviour of the LSCC composites has been explored under air and argon atmospheres in terms of evolution of the chemical composition at the grain's surface and phase interfaces, formation of oxygen vacancies and thermochemistry of this process. Reversible red-ox behaviour was observed in LSCCx with x = 8–37 most probably due to an observed high surface concentration of Co cations, that can be easily involved in the reduction/re-oxidation cycle. The increase in the surface concentration of Ce4+ cations together with the decrease in surface concentration of Co cations seems to result in the differences in the reduction and oxidation behaviour under air in LSCCx with x = 57–76. Formation of oxygen vacancies in LSC, LSCC02 and LSCCx with x = 5–76 in air was not accompanied by any distinct thermal events. This process becomes more endothermic with further increase in oxygen nonstoichiometry (δ) above certain values: δ > 0.08 in LSC, δ > 0.13 in LSCC02, and LSCC with x = 5–76. The LSCCx with x = 5–37 and with x = 57–76 show slightly different reduction behaviour under a(o2) = 7.4 × 10−5. In the composites with a relatively low CeO2 content, the extent of the reduction is proportional to the Co content in a composition, whereas the reduction of the LSCCx with x = 57–76 was more significant than expected. The changes in the enthalpy of oxygen vacancy formation and the kinetics of reduction have been discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c) and modified ceria with fluorite structure (Fm
c) and modified ceria with fluorite structure (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m). The cross-dissolution of La, Sr, Co and Ce
m). The cross-dissolution of La, Sr, Co and Ce ![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c) to cubic (Pm
c) to cubic (Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) within the perovskite phase. Red-ox behaviour of the LSCC composites has been explored under air and argon atmospheres in terms of evolution of the chemical composition at the grain's surface and phase interfaces, formation of oxygen vacancies and thermochemistry of this process. Reversible red-ox behaviour was observed in LSCCx with x = 8–37 most probably due to an observed high surface concentration of Co
m) within the perovskite phase. Red-ox behaviour of the LSCC composites has been explored under air and argon atmospheres in terms of evolution of the chemical composition at the grain's surface and phase interfaces, formation of oxygen vacancies and thermochemistry of this process. Reversible red-ox behaviour was observed in LSCCx with x = 8–37 most probably due to an observed high surface concentration of Co 

 Please wait while we load your content...
Please wait while we load your content...