The peculiar structural behaviour of β-Na0.33V2O5 upon electrochemical lithium insertion
Abstract
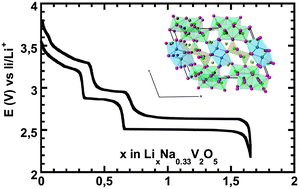
The structural behaviour of the β-Na0.33V2O5 active material in a composite electrode is determined during discharge in the 3.8/2.2 V potential range (0 ≤ x < 1.66 in LixNa0.33V2O5) using

 Please wait while we load your content...
Please wait while we load your content...