Efficient water splitting over Na1−xKxTaO3 photocatalysts with cubic perovskite structure
Abstract
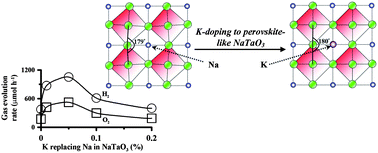
This study improved the photocatalytic activity of NaTaO3 by replacing some Na ions in the 12-coordinate sites with larger K ions. Na1−xKxTaO3 photocatalysts of x = 0–0.2 were synthesized by the sol–gel method. K-doping at x = 0.05 resulted in rectifying the distorted perovskite NaTaO3 to a pseudo-cubic phase as well as significantly promoting the photocatalytic activity. The 180° bond angle of Ta–O–Ta in the pseudo-cubic phase may facilitate the separation of photogenerated charges for effective

 Please wait while we load your content...
Please wait while we load your content...