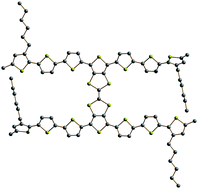
Electronic, redox and charge transport properties of an unusual hybrid structure: a bis(septithiophene) bridged by a fused tetrathiafulvalene (TTF)†‡
Abstract
A hybrid
- This article is part of the themed collection: Celebrating the 70th birthday of Professor Fred Wudl

 Please wait while we load your content...
Please wait while we load your content...