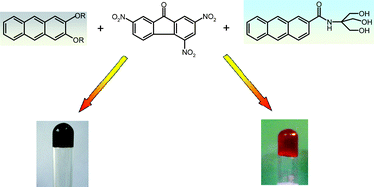
This article describes two-component charge-transfer interaction mediated organogels (CT-gels) derived from anthracene carboxamides obtained from 2-amino-2-hydroxymethyl-1,3-propanediol (TRIS), and 2,3-dialkoxyanthracenes as donors, with 2,4,7-trinitrofluorenone (TNF) as the common acceptor. We demonstrate the versatility of TNF as an electron acceptor in the formation of these gels. The effect of subtle changes in the donor structure on the gelation ability has been investigated by varying the alkyl chain length in the dialkoxyanthracene donors, and by varying the position of the TRIS substituent in the anthracene carboxamide donors. Distinct differences have been observed in the nature of the CT-gels based on these two kinds of anthracene donors. It has been reported in the literature that 2,3-dialkoxyanthracenes form gels on their own in various aliphatic hydrocarbons and alcohols for linear alkyl chains bearing at least 6 carbon atoms (C6). In the present study, it is shown that the CT-complex of these molecules with TNF is able to gel many alcoholic and a few hydrocarbon solvents. Also, in the presence of TNF, the 2,3-dialkoxyanthracenes (C4–C5) which were non-gelators on their own at ambient temperatures, form CT-gels in a number of alcohols. The other series of gelators discussed, the anthracene carboxamides, require the mandatory presence of TNF to form gels. This donor–acceptor complex forms gels in various aliphatic alcohols. Interestingly, the formation of these CT-gels requires rapid cooling in most of the cases. Thermal stability studies with both types of CT-gels indicate an optimum stoichiometry of 1 : 1 between the donor and the acceptor. Dynamic rheological experiments reveal these gels as viscoelastic soft materials, with the mechanical strength of these gels depending on the amount of TNF present. This provides a means to tune the strength of the gel by varying the doping concentration of the acceptor.

 Please wait while we load your content...
Please wait while we load your content...