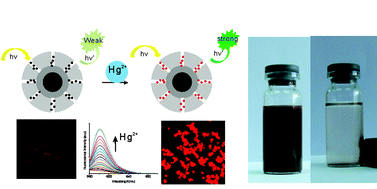
A novel, “all-in-one”, multifunctional microsphere with a fluorescent mesoporous silica shell (Rhodamine B coordinate receptor inside) and a magnetic core (Fe3O4) has been successfully fabricated using a sol–gel method and small molecular (CTAB) surfactants as structure-directing agents. At the same time, they were examined for environmental protection applications to detect, adsorb and remove Hg2+ in aqueous solution. The prepared nanocomposite microspheres were fluorescent, mesoporous, and magnetizable, with a diameter of 300–450 nm, a surface area of 600 m2 g−1, a pore size of 2.5 nm, and a saturation magnetization of 27.5 emu g−1. These multifuctional microspheres showed excellent fluorescence sensitivity and selectivity towards Hg2+ over other metal ions (Na+, Mg2+, Mn2+, Co2+, Ni2+, Zn2+, Cd2+, Ag+, Pb2+ and Cu2+). Upon the addition of Hg2+, an overall emission change of 16-fold was observed, and the detection limit of Hg2+ was as low as 10 ppb. The adsorption process of Hg2+ on the microspheres was well described by the Langmuir equation. The equilibrium can be established within five minutes and the adsorption capacity was 21.05 mg g−1. The concentration of Hg2+ ions can be reduced to less than 0.05 ppm and the used microspheres can be easily separated from the mixture by adding an external magnetic field. These results suggest that these “all-in-one” multifunctional nanocomposites are potentially useful materials for simultaneously rapidly detecting and recovering dangerous pollutants in aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...