Room-temperature discotic liquid crystals based on oligothiophenes—attached and fused triazatruxenes†
Abstract
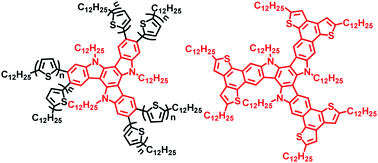
A new class of discotic liquid crystals based on oligothiophenes - attached and fused triazatruxenes, were synthesized in high yields. The synthesis was carried out first by microwave-assisted six-fold Stille cross-coupling reactions to yield the hexakis-oligothiophenes - substituted triazatruxenes TAT-T1 and TAT-T2 and followed by successful oxidative cyclodehydrogenation of TAT-T1 to afford the planar TAT-T1-C with an extended core. Their optical properties, electronic properties, thermal behavior and

 Please wait while we load your content...
Please wait while we load your content...