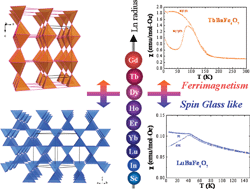
The investigation of the “114” ferrites has allowed seven new compounds of LnBaFe4O7 formula to be synthesized: the compounds LnBaFe4O7 (Ln = Dy, Ho, Er, Yb, Lu) with a cubic symmetry (a ≈ 8.9 Å) and the oxides TbBaFe4O7 and GdBaFe4O7 with hexagonal symmetry (a ≈ 6.34 Å and c ≈ 10.38 Å). Moreover, two other oxides, out of the lanthanide series, InBaFe4O7 and ScBaFe4O7, were also obtained with cubic symmetry. The relative stability of one structure with respect to the other is explained in terms of the relative sizes of the Ln3+ (or In3+, Sc3+) cations with respect to the Fe2+/Fe3+ cations for tuning the triangular and kagome layers in two different ways. The magnetic properties of these oxides show that all the compounds with cubic symmetry can be described as spin-glass like in agreement with their [Fe4]∞ tetrahedral framework similar to pyrochlores, whereas the hexagonal phases, (Tb/Gd)BaFe4O7, exhibit ferrimagnetism, like CaBaFe4O7 but with a much smaller TC = 160 K. It is found that these symmetries are responsible for the two different types of magnetic frustration.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...