Hydrothermal synthesis of LnMnO3 (Ln = Ho–Lu and Y): exploiting amphoterism in late rare-earth oxides†
Abstract
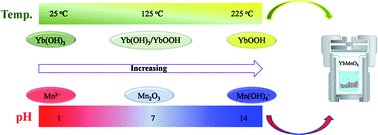
The reactivity of Mn2O3 and late rare-earth sesquioxides in alkaline aqueous solution affords a high-yield formation of rare-earth manganites, LnMnO3 (Ln = Ho–Lu and Y). The yield of the products depends significantly on the pH, which determines the solubility of the
- This article is part of the themed collection: Dedicated to Professor C. N. R. Rao

 Please wait while we load your content...
Please wait while we load your content...