A thermodynamically stable layered double hydroxide heterostructure
Abstract
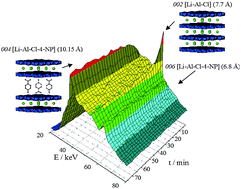
A novel heterostructure was synthesised via the intercalation of 4-nitrophenolate (4-NP) into [LiAl2(OH)6]Cl·xH2O ([Li-Al-Cl]). Its interlayer spaces are alternatively occupied by 4-NP and chloride ions. The intercalate is structurally analogous to staging intermediates reported previously. However, data obtained from time-resolved in situ energy-dispersive

 Please wait while we load your content...
Please wait while we load your content...