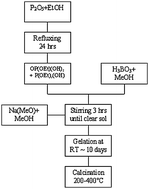
Glasses in the system 40(P2O5)–x(B2O3)–(60 − x)(Na2O) (10 ≤ x ≤ 25 mol%) were prepared by the sol–gel technique. A mixture of mono- and diethylphosphates was used as precursor for P2O5, boric acid and sodium methoxide were used as source compounds for B2O3 and Na2O, respectively. The dried gels obtained were heat treated at 200, 300 and 400 °C. Structural development occurring during heat treatment and changes with composition were investigated using X-ray diffraction, thermal analysis, infrared spectroscopy, 11B and 31P solid state NMR. Systems with x = 20 and x = 25 mol% are amorphous up to 400 °C, whereas systems with lower B2O3 content are partially crystalline. This work extends sol–gel preparation of amorphous borophosphate systems having P2O5 as the main component.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...