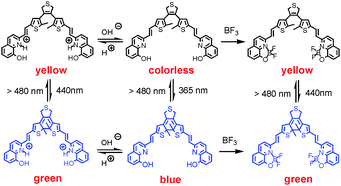
A multi-addressable switching system has been constructed by employing photochromic diarylethene 1a, which is obtained by coupling photochromic 3,4-bis(5-formyl-2-methylthien-3-yl)-2,5-dihydrothiophene with 8-hydroxy-2-methylquinoline, as model compound. It is found that 1a exhibits ring-opening and ring-closing photoisomerization with UV/Vis light irradiation. Addition of trifluoroacetic acid to solution of 1a produces protonated diarylethene 2a, and 2a also performs photochromic behavior with distinguished color change. Both protonated ring-open isomer 2a and ring-closed isomer 2b reverse back to 1a and 1b by neutralization with base solution. Besides, a complex diarylethene 3a is formed when boron trifluoride diethyl etherate is added to a solution of 1a, and it is found that 3a also undergoes excellent photoisomerization.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...