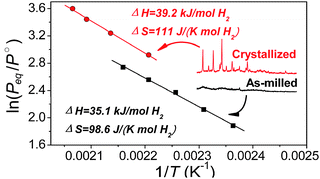
Mg(NH2)2–2LiH is an attractive system because of its high reversible hydrogen capacity (∼5.6 wt%) and suitable thermodynamic parameters that allow operation below 100 °C. However, a relatively high kinetic barrier in the hydrogen desorption blocks its application at low temperature. In this work, a small amount of additive, triphenyl phosphate (TPP), was introduced and its effects on hydrogen desorption/absorption in the Mg(NH2)2–2LiH system were studied. Experimental results showed that TPP can prevent aggregation/crystallization during the cycling tests and thus achieve an enhanced kinetic performance. Complete dehydrogenation and hydrogenation can be successfully carried out at temperatures below 150 °C. Moreover, a significant reduction of the entropy change of hydrogen desorption (ΔSdes) was found in the TPP-doped system compared with the pristine Mg(NH2)2–2LiH system, thought to be due to the persistence of amorphous Mg(NH2)2 in the TPP-doped sample during dehydrogenation and hydrogenation cycling, thereby greatly affecting the equilibrium hydrogen pressure.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...