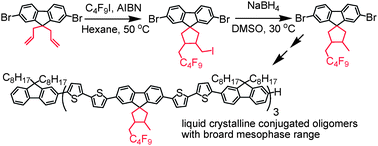
3′-Nonafluorobutylmethyl-4′-methyl-spiro[cyclopentyl-9,1′]fluorenes were successfully synthesized via tandem radical-addition reactions between 9,9-diallylfluorenes and perfluorobutyl iodide in the presence of a radical initiator followed by reduction under mild conditions. Single crystal analysis indicates that two substituents at 3,4-positions of cyclopentane are in a maleinoid form. Accordingly, four oligo(fluorene-co-bithiophene)s with the same molecular length of ∼10 nm (7 fluorene units and 12 thiophene units) containing one to three novel spiro-fluorene units were synthesized. The introduction of the spiro-fluorene units results in noticeable enhancement of both glass transition temperature (Tg) and clearing point temperature (Tc) of the oligomers, but has little effect on their photophysical properties. Exchanging three 9,9-dioctylfluorene units with 3′-nonafluorobutylmethyl-4′-methyl-spiro[cyclopentyl-9,1′] fluorene units results in a 37 °C enhancement of Tg and a 61 °C enhancement of Tc. All these results indicate that this new spiro-fluorene unit is an attractive building block for liquid crystalline conjugated polymers/oligomers with both high Tg and Tc.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...