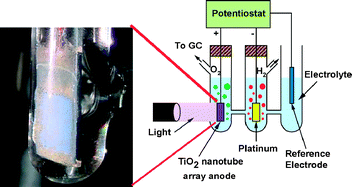
Described is the synthesis of TiO2 nanotube array films by anodization of Ti foil in HCl electrolytes containing different H2O2 concentrations. Highly ordered nanotube arrays up to 860 nm in length, 15 nm inner pore diameter, and 10 nm wall thickness were obtained for one hour anodizations using a 0.5 M HCl aqueous electrolyte containing 0.1–0.5 M H2O2 concentrations for anodization potentials between 10–23 V. The use of ethylene glycol as the electrolyte medium significantly alters the anodization kinetics and resulting film morphologies; nanotube bundles several microns in length achieved for anodization potentials between 8 V and 18 V in only a few minutes. The nanotube arrays obtained from the ethylene glycol electrolytes show relatively higher photocurrents, ≈0.8 mA cm−2 under AM 1.5. Under 100 mW cm−2 AM 1.5 illumination a 500 °C annealed 1 cm2 nanotube array sample, obtained by anodization of a Ti foil sample in ethylene glycol + 0.5 M HCl + 0.4 M H2O2 electrolyte, demonstrates a hydrogen evolution rate of approximately 391 μL h−1 by water photoelectrolysis, time-power normalized evolution rate of 3.9 mL W−1 h−1, with water splitting confirmed by the 2 : 1 ratio of evolved hydrogen to oxygen.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...