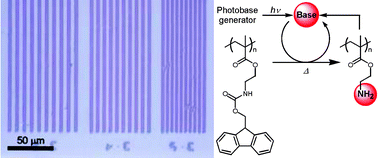
We describe highly sensitive photopolymers consisting of a photobase generator (PBG), a base-sensitive polymer, and a base amplifier that decomposed autocatalytically to release an amine molecule which resulted in the enhancement of base-catalyzed reactions of the base-sensitive polymer. Low molecular weight base molecules produced from the base amplifiers were widely diffused in the polymer film, resulting in the reduction of resolution power in the manner of “air infection” of low molecular weight basic species, although the photosensitivity was enhanced. To improve the performance, we have designed a novel base-sensitive polymer (1) tethering base-amplifying units that autocatalytically transform into amino groups attached to the polymer backbone. A film of 1 sensitized with PBG was decomposed autocatalytically by UV irradiation and subsequent heat treatment. This means that the base proliferation reaction of 1 proceeded regardless of its polymer structure. In addition, a film of 1 sensitized with PBG provided a positive working photoresist developable with an acidic aqueous solution and showed much higher photosensitivity when compared with conventional chemically amplified photoresists comprising PBG and base-labile polymers. Moreover, the use of base-amplifying polymer 1 led to prevention of “air infection”, and improvement in the resolution power of photopolymers relying on base proliferation reactions when compared with base-sensitive photopolymers coupled with low molecular weight base amplifiers.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...