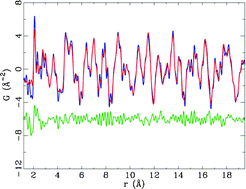
The local environment and short-range ordering of Li(Ni0.5Mn0.5)O2, a potential Li-ion battery positive electrode material obtained via an ion-exchange route from Na(Ni0.5Mn0.5)O2, were investigated by using a combination of 6Li Magic Angle Spinning (MAS) NMR spectroscopy and neutron Pair Distribution Function (PDF) analysis, associated with Reverse Monte Carlo (RMC) calculations. 6Li MAS NMR experiments on Li(Ni0.5Mn0.5)O2 showed that there are almost no Li ions in the transition metal layers. Neutron diffraction data for the precursor Na(Ni0.5Mn0.5)O2 indicated that there is no Na/Ni disorder and that the material is perfectly layered. Neutron PDF analysis of Li(Ni0.5Mn0.5)O2 and Na(Ni0.5Mn0.5)O2 revealed differences in the local transition metal arrangements between those present in the ion-exchanged material and its precursor, and those found in the cathode material synthesized directly from hydroxide starting materials. Large clusters of 3456 atoms were built to investigate cation ordering. Reverse Monte Carlo results, for both the Na and Li-containing compounds, showed a non-random distribution of Ni and Mn cations in the transition metal layers: in the first coordination shell, Ni atoms are on average close to more Mn ions than predicted based on a random distribution of these ions in the transition metal layers. Analysis of the number of Ni/Ni, Mn/Mn and Ni/Mn pairs in the second coordination shell revealed that the Ni and Mn cations show a clear preference for ordering in zigzags rather than in chains.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...