Abstract
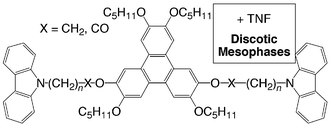
The synthesis of 13 discotic mesogens is described in which the well-known hexakis(pentyloxy)triphenylene liquid crystalline material has been chemically modified to incorporate one, two, three and six carbazole moieties. These modifications have been achieved by the alkylation or esterification of mono-, di-, tri- and hexa-hydroxytriphenylene derivatives with alkyl bromides and carboxylic acids incorporating the carbazole moiety. The pure compounds are not liquid crystalline in nature but when doped with TNF, hexagonal columnar mesophases are induced, as shown by DSC, OPM and X-ray diffraction. These mesophases exist below room temperature. The mesophase clearing temperatures are dependent on several factors including the chain length separating the carbazole moiety from the triphenylene core, and the nature of the ether or ester linkage, and the degree of TNF doping. The data suggest that the most stable mesophases (highest clearing temperatures) are formed when
a 2 ∶ 1 complex is formed between the carbazole derivatives and the TNF, respectively. The correlation length obtained from X-ray diffraction reveals that the columnar order for one of the ether derivatives decreases, whereas the correlation length increases for one of the ester derivatives. This result suggests that the TNF is not only partaking in π–π noncovalent bonding interactions, but also in polar interactions with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. Such mesogenic carbazole derivatives may have advantageous photorefractive properties over the amorphous polymeric materials.
O bond. Such mesogenic carbazole derivatives may have advantageous photorefractive properties over the amorphous polymeric materials.
- This article is part of the themed collection: Molecular topology in liquid crystals: Materials Discussion 4

 Please wait while we load your content...
Please wait while we load your content...