A unified approach to mechanistic aspects of photochemical vapor generation
Abstract
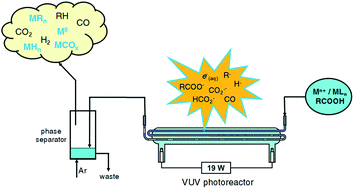
A unified approach to the mechanism of photochemical vapor generation (PVG) of metal, semimetal and halogen elements is proposed. Evidence is drawn from extensive parallel literature devoted to radical-based reactions involving metal ions in aqueous and carboxylate-containing media undertaken with flash photolysis and pulse radiolysis, in addition to classical photolysis and the impact of semiconductor mediated metal ion reduction (removal) from solution. A general model of homogeneous redox reactions emerges, driven by several widely recognized ubiquitous powerful reducing radicals, notably e(aq)−, H˙, R˙ and CO2˙−. In each of the metal ion based systems, reduction to the elemental state is thermodynamically feasible, followed by trapping with excess concentrations of H˙, H3C˙ and CO arising from concurrent VUV photolysis and concomitant decarboxylation of formic and/or acetic acid to yield the identified product. For complex oxyanions ([MxOy]n−), such as those of Se(VI), slow kinetics of reduction to Se0 can be overcome by the intervention of heterogeneous catalysis based on TiO2 or other “designer” semiconductor photocatalysts that facilitate rapid reactions on their surfaces, to which the metal species have adsorbed. While applications of photocatalysts are more cumbersome, they provide an alternative means of generating CO2˙− and other reducing radicals (derived from added carboxylic acids or alcohols serving as sacrificial oxidants), at wavelengths higher than frequently used VUV and UV-C sources, depending on the band gap of the semiconductor and the redox potential of its conduction band. As with most redox processes, the reduction potential in the system may be pH dependent, manifesting itself in several ways, including the degree of complexation in some metal-carboxylate systems, the extent of surface adsorption of species onto catalytic surfaces, the Nernstian potential of the redox couple involved and the degree of ionization of the HCOO˙−/CO2˙− couple (hence its redox potential). In the case of the halogens, either direct photochemical generation of X˙ followed by radical–radical coupling with H3C˙ yields the XCH3 product, or the intervention of transition metal halides which permit radical halogen-transfer from such species as FeX2+ find utility for photosynthesis of XCH3.
- This article is part of the themed collections: Recent Review Articles and Community Leaders: Alfredo Sanz-Medel


 Please wait while we load your content...
Please wait while we load your content...