Lewisite exposure biomarkers in urine by liquid chromatography – inductively coupled plasma tandem mass spectrometry: with an accelerated matrix-matched stability study†
Abstract
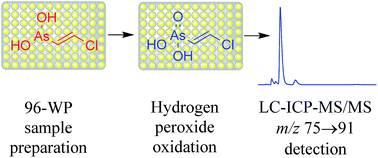
A simple and robust LC-ICP-MS/MS method is described for quantitative analysis of human urine for (2-chlorovinyl)arsonic acid (CVAOA), a metabolite of Lewisite. This method oxidizes (2-chlorovinyl)arsenous acid (CVAA) with the addition of hydrogen peroxide to measure total Lewisite-1 metabolites as CVAOA, with m/z 75 → 91 detection specific for arsenic. The percentage of CVAA to CVAOA is clinically insignificant, because the amount of CVAA conversion to CVAOA is dependent upon residence time in the body. Once excreted into the urine, conversion of CVAA to CVAOA is dependent upon temperature and oxidative potential of the urine. The method also allowed for qualitative analysis for bis(2-chlorovinyl)arsinic acid (BCVAOA) and (1-chlorovinyl)arsonic acid (gem-CVAOA), minor Lewisite metabolites. Traditional methods have ignored these minor metabolites; the bis-metabolites can comprise ∼30% of total Lewisite metabolites from chemical munitions and must be accounted for in the exposure measurement. The ion-pairing chromatography method results in a 5.73 min injection-to-injection cycle time with adequate retention (k′ = 2.9) of CVAOA. The weighted (1/x2) linear least squares regression results have correlation coefficients (r2 > 0.998) for the clinically relevant calibration range of 50–3500 μg L−1. The selectivity of the method is measured by chromatographic resolution from other common arsenic compounds that may interfere with the analysis. The 96-well plate preparation of 0.1 mL sample of human urine results in a method detection limit of 2.2 μg L−1. Quantitative results from proficiency testing specimens demonstrate the accuracy (−7.1 to +4.3%) of the method. Quality control data demonstrate inter-analyst precise (3.1 to 3.3%) quantitative results of the method. An accelerated Arrhenius matrix-matched stability study demonstrates Lewisite metabolites are stabile in urine far greater than a year. The trivalent arsenic, CVAA oxidation half-life is estimated at normal body temperature in vitro at 6.2 days. The combined sample preparation and analysis portions of this emergency response method have a throughput of 250 samples per day.

 Please wait while we load your content...
Please wait while we load your content...