Chemical halide vaporization as a sample introduction technique for atomic spectroscopy: significance of its kinetic mechanism
Abstract
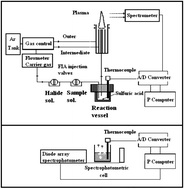
Chemical vaporization with halides, as a means of sample introduction in atomic spectroscopy, has been discussed taken into account the kinetics of the processes. The studied vaporizations involved those produced by As(III), Ge(IV), Sb(III–V) and Se(IV–VI) with chloride, bromide and fluoride ions. Vaporization was achieved using a systematic procedure: elements were reacted with aqueous halide solutions in concentrated sulphuric acid and were characterized by: the emission peaks in ICP-OES, temperature and molecular absorbance of reacting solutions. This last parameter allowed the kinetics of vaporizations to be determined. According to these results clear differences were observed between the vaporizations. Thus, a first type of halides, formed by Ge(IV)–chloride, Se(IV)–bromide and As(III)–fluoride species, could be identified by their large rate constant, Kv, sensitive determinations by ICP-OES and appearance time in the range of 1–2 min. The second type was characterized by Kv values smaller than 0.2 min−1, a sensitivity decrease with respect to the first group and the appearance time in ICP-OES was longer than 2.0 min. On the other hand, an outstanding behavior was presented by species such of As(III)–chloride, Se(VI)–bromide and Sb(V)–bromide. They were vaporized rapidly and the peaks appeared 1 min after the reaction onset. The optimum reaction conditions were clearly influenced by the halide composition. Thus chloride species were vaporized at medium to high chloride concentrations whereas bromide and fluoride species required low halide concentrations. Vaporizations with a significant analytical potential were evidenced for species formed by Ge(IV) and As(III) with chloride and Sb(V) and Se(VI) with bromide. Also As(III)–fluoride species retained some of these characteristics.

 Please wait while we load your content...
Please wait while we load your content...