Studies of structure and phosphorylation of tau protein using high resolution mass spectrometry†
Abstract
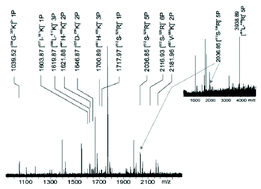
Tau as a
- This article is part of the themed collection: Second Asia-Pacific Winter Conference on Plasma Spectrochemistry, Bangkok, Thailand

 Please wait while we load your content...
Please wait while we load your content...