Phosphatidylcholine in the groove of endothelial cell protein C receptor (EPCR) regulates EPCR conformation and protein C recognition†
Abstract
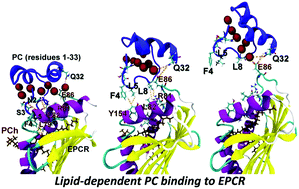
Endothelial cell protein C receptor (EPCR), the cellular receptor for protein C (PC), facilitates PC activation through the thrombin/thrombomodulin complex and regulates thrombin generation. Under pathophysiological conditions like sepsis, the interactions between EPCR and PC become impaired. Previous studies have demonstrated that the EPCR contains a phospholipid in the antigen-binding groove that is responsible for the structural stability of the EPCR and for PC recognition. However, an understanding at the atomic level during ligand recognition is not fully developed. Molecular dynamics simulations along with potential of mean force (PMF) calculations were carried out in order to provide molecular insight into the dynamics and free energies of EPCR–PC in the absence/presence of phospholipid, namely lysophosphatidylcholine (lysoPCh) and phosphatidylcholine (PCh) in the antigen-binding groove of the EPCR. Our data reveal that the presence of lipid maintains the optimal conformation of the EPCR for PC binding. PMF data further suggest that the PCh system is the most stable in comparison with the other systems (lysoPCh and no lipid). With regards to the two hydrophobic tails of PCh, one lipid tail regulates EPCR conformation while the other promotes ligand recognition by interacting with the keel residue (Phe-4) of PC. Due to the lack of one hydrophobic tail for the lysoPCh system, the EPCR conformation is retained but the affinity of the EPCR towards the ligand (PC) is reduced. Our studies for the first time explore the possible mode of ligand recognition by the EPCR via the involvement of phosphatidylcholine within its hydrophobic groove. The present work provides insight into PCh-dependent ligand recognition and hence regulation of the protein C/EPCR complex formation.


 Please wait while we load your content...
Please wait while we load your content...