Endothelial monolayer permeability under controlled oxygen tension†
Abstract
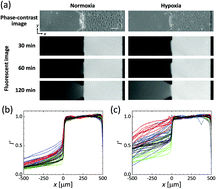
Endothelial permeability has been extensively investigated in the context of pathologies such as cancer and also in studies of drug delivery from the circulation. Hypoxia is a critical regulator of endothelial cell (EC) behavior and affects the barrier function of endothelial linings, yet its role has been little studied. This paper reveals the effect of hypoxia on the permeability of an EC monolayer by cellular experiments using a microfluidic device and a conventional cell culture dish. Human umbilical vein endothelial cells (HUVECs) were seeded into one microfluidic channel, creating an EC monolayer on each vertical surface of a collagen gel confined to a central chamber. Oxygen tension was regulated to produce normoxic (21% O2) or hypoxic (3% O2) conditions by the supply of gas mixtures of oxygen, carbon dioxide, and nitrogen at predefined ratios into channels fabricated into the device. Permeability of the EC monolayer quantified by analyzing diffusion of fluorescence-labelled dextrans into the collagen gel increases with barrier function loss by 6 hour hypoxic exposure, showing 11-fold and 4-fold increases for 70 kDa and 10 kDa dextrans, respectively, on average. Consistent with this, subsequent immunofluorescent staining and separate western blot analysis of HUVECs on a culture dish demonstrate loose cell–cell adhesion resulting from internalization of VE-cadherin under hypoxia. Thus, hypoxic stress increases endothelial permeability by altering cell–cell junction integrity.


 Please wait while we load your content...
Please wait while we load your content...