Protein self-assembly following in situ expression in artificial and mammalian cells†
Abstract
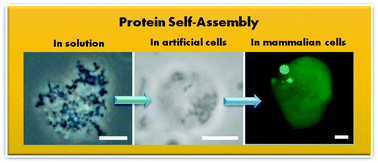
The self-assembly of proteins has been widely studied in controlled in vitro conditions, and more recently in biological environments. The self-assembly of proteins in biology can be a feature of the pathogenesis of protein condensation disease, or can occur during normal physiological function, for example during the formation of intracellular non-membrane bound organelles. To determine the mechanisms for the assembly process fully, controlled in vitro experiments using purified protein solutions are often required. However, making direct connections between insights gathered from controlled experiments and those in complex biological environments remains a challenge. Using the P23T mutant of human γD-crystallin, a protein associated with congenital cataract, we have demonstrated that the equilibrium solubility boundary and solution behavior measured using phase diagrams of purified protein solutions is consistent with the assembly of the protein expressed in cell-free expression medium in artificial cells (without fluorescent labelling) and condensates formed in mammalian cells, thereby directly connecting in vitro measurements with those performed under physiological conditions.


 Please wait while we load your content...
Please wait while we load your content...