Allosteric effects of ATP binding on the nucleotide-binding domain of a heterodimeric ATP-binding cassette transporter†
Abstract
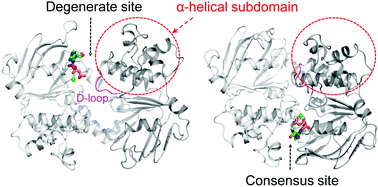
ATP-binding cassette (ABC) exporters mediate vital transport of a variety of molecules across the lipid bilayer in all organisms. To explore the allosteric effect of ATP binding at the asymmetric ATPase sites, molecular dynamics simulations were performed on the nucleotide-binding domains (NBDs) of a heterodimeric exporter TM287/288 in 4 different ATP-bound states. The results showed that ATP bound at the degenerate site can maintain a semi-open conformation of NBD1–NBD2, which may be defective in ATP hydrolysis. By contrast, when bound at the consensus site, ATP can induce an intra-domain rotation of the α-helical subdomain towards the RecA-like subdomain of NBD2 at the degenerate site. The rotation of the α-helical subdomain rearranged the hydrogen bond networks at the NBD1–NBD2 interface, induced a significant conformational change in the D-loop at the degenerate site and inter- and intra-domain communications at both sites, and eventually elicited dimerization of NBD1–NBD2. These findings indicate that the asymmetric ATPase sites of the heterodimeric exporter are structurally and functionally distinct.

 Please wait while we load your content...
Please wait while we load your content...