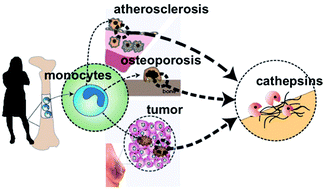
Patient-to-patient variability in disease progression continues to complicate clinical decisions of treatment regimens for cardiovascular diseases, metastatic cancers and osteoporosis. Here, we investigated if monocytes, circulating white blood cells that enter tissues and contribute to disease progression by differentiating into macrophages or osteoclasts, could be useful in understanding this variability. Monocyte-derived macrophages and osteoclasts produce cysteine cathepsins, powerful extracellular matrix proteases which have been mechanistically linked to accelerated atherosclerotic, osteoporotic, and tumor progression. We hypothesized that multivariate analysis of temporal kinase activation states during monocyte differentiation could predict cathepsin proteolytic responses of monocyte-derived macrophages and osteoclasts in a patient-specific manner. Freshly isolated primary monocytes were differentiated with M-CSF or RANKL into macrophages or osteoclasts, respectively, and phosphorylation of ERK1/2, Akt, p38 MAPK, JNK, c-jun, and IκB-α were measured at days 1, 3, 6, and 9. In parallel, cell diameters and numbers of nuclei were measured, and multiplex cathepsin zymography was used to quantify cathepsins K, L, S, and V activity from cell extracts and conditioned media. There was extensive patient-to-patient variability in temporal kinase activation states, cell morphologies, and cathepsin K, L, S, and V proteolytic activity. Partial least squares regression models trained with temporal kinase activation states successfully predicted patient-specific morphological characteristics (mean cell diameter and number of nuclei) and patient-specific cathepsin proteolytic activity with predictability as high as 95%, even with the challenge of incorporating the complex, unknown cues from individual patients' unique genetic and biochemical backgrounds. This personalized medicine approach considers patient variability in kinase signals to predict cathepsin activity. Such analyses may provide beneficial tools for personalized kinase and protease inhibitor therapies for tissue destructive diseases.

 Please wait while we load your content...
Please wait while we load your content...