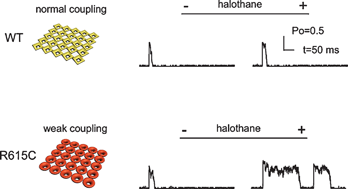
Functional coupling between clustered membrane receptors has been identified as a novel mechanism to improve signaling performance in a number of physiological processes. The potential role of defective inter-receptor coupling in the pathogenesis of disease, however, has not previously been explored. Ryanodine receptors (RyRs), the primary calcium release channel of muscle, usually form ordered two-dimensional arrays in the sarcoplasmic reticulum membranes. Mutations in RyRs are known to cause a number of severe diseases both in skeletal muscle and in heart. Here we present a model demonstrating how impaired functional coupling between neighboring mutant RyR1R615C channels may contribute to the pharmacogenetic skeletal muscle sensitivity, malignant hyperthermia (MH). We find that purified RyR1R615C from MH susceptible porcine skeletal muscle shows significantly reduced oligomerization when compared to RyR1WT, indicating a potential loss of intrinsic intermolecular control. The MH-triggering volatile anesthetic, halothane, activates RyR1R615C and RyR1WT to a similar extent, using [3H]ryanodine binding as a measure of activation. Modeling RyR1 array function with parameters modified to simulate the loss of functional inter-RyR coupling recapitulates the MH molecular phenotype-RyR1 channels leaky to Ca2+ at rest and long open-times following exposure to halothane. Our work suggests that a defect in inter-RyR1 coupling is a novel direction for research into the pathogenesis of MH.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...