Effect of solute polarity on extraction efficiency using deep eutectic solvents†
Abstract
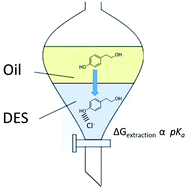
While ionic liquids and deep eutectic solvents, DESs, have been extensively used for natural product extraction relatively little is known about the factors affecting extraction efficiency. In this study, 7 simple solutes are extracted into 4 DESs at two temperatures and the thermodynamics of phase transfer are determined. It was found that solutes which are able to form hydrogen bond are more successfully extracted into the DES phase from cyclohexane. For less polar solutes, the extracting DES has a more pronounced effect on extraction efficiency with liquids of a lower surface tension being more effective. With polar solutes the effect of the DES is less pronounced. It is shown that the Gibbs energy of extraction is proportional to the pKa of the solute demonstrating the importance of hydrogen bonding in solute partition. The study was extended to 5 phenolic compounds commonly found in olive oil and again the extraction efficiency was shown to be related to the solute pKa. The green metrics for the extraction of a range of solutes were determined and shown in some cases to be superior to molecular solvents. The energy consumption of extraction was shown to be comparatively small even when mechanically assisted.


 Please wait while we load your content...
Please wait while we load your content...