Sustainable access to sulfonic acids from halides and thiourea dioxide with air†
Abstract
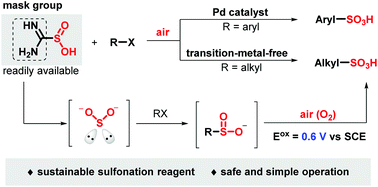
A sustainable and mild one-step strategy is explored for the synthesis of aryl and alkyl sulfonic acids using a facile combination of halides and sulfur dioxide surrogates under air. The cheap industrial material thiourea dioxide was employed as an eco-friendly and easy-handling sulfur dioxide surrogate, while air was used as a green oxidant. Both aryl and alkyl sulfonic acids were obtained under transition metal-catalyzed or transition metal-free conditions. Mechanistic studies demonstrated that sulfinate was involved as an intermediate in this transformation. Notably, this protocol has been applied to the late-stage sulfonation of the drugs naproxen, isoxepac and ibuprofen.


 Please wait while we load your content...
Please wait while we load your content...