A molten calcium carbonate mediator for the electrochemical conversion and absorption of carbon dioxide†
Abstract
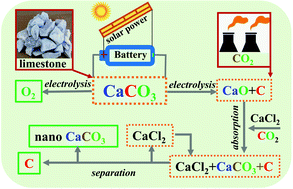
High-temperature molten salts are an excellent electrolyte to bring about redox reactions at a rapid rate without using rationally designed nano-structured catalysts. However, the large-scale electrolyzer is constrained by the use of expensive and resource-deficient lithium salts. Using Earth-abundant CaCO3 releases the pressure of using strategic lithium resources, but the low solubility of CaO in molten carbonates disables the capability of capturing CO2. In addition, the separation of carbon from water-insoluble CaO and CaCO3 consumes a large amount of acids. To tackle these challenges, we report a CaCO3-containing molten carbonate electrolyzer to prevent the use of lithium salts, and a molten CaCl2 dissolver to separate carbon from CaO that is soluble in molten CaCl2 and can capture CO2 by carbonization. More importantly, we develop a salt-soluble-to-water-insoluble approach for producing ultrafine CaCO3 using molten salt as a soft template. Overall, this study opens a pathway to use cheap and Earth-abundant molten CaCO3 as a mediator to convert CO2 to oxygen at a cost-effective inert anode, with value-added carbon at the cathode, and ultrafine CaCO3 through a salt-to-solution process.


 Please wait while we load your content...
Please wait while we load your content...