Hydrothermal carbonization of sewage sludge: effect of inorganic salts on hydrochar's physicochemical properties†
Abstract
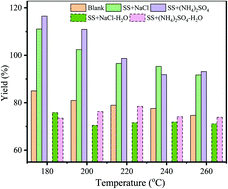
NaCl and (NH4)2SO4 were selected as typical inorganic salts (10 wt% addition) in order to analyze their effects on the hydrothermal carbonization (HTC) of sewage sludge (SS). Results indicated that both salts could be adsorbed on the surface of hydrochar, resulting in amounts of hydrochar increasing and potentially serious nitrogen and chlorine issues upon employing SS hydrochar as solid fuel. NaCl and (NH4)2SO4 could act as catalyst to promote hydrolysis and carbonization of SS, with (NH4)2SO4 participating in the carbonization of SS. During combustion, Cl decreased the thermal decomposition temperature of hydrochar. The reported results can provide information to optimize the use of chloride salts and NH4+ concentration for increasingly efficient HTC processes.


 Please wait while we load your content...
Please wait while we load your content...