Abstract
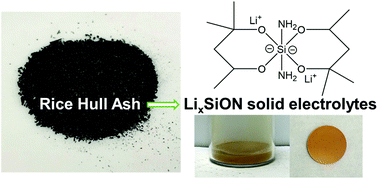
A set of LixSiON (x = 2, 4, 6) polymer precursors to a novel solid-state electrolyte system were synthesized starting from rice hull ash (RHA), an agricultural waste, providing a green route towards the assembly of all solid-state batteries (ASSBs). Silica, ∼90 wt% in RHA, can be catalytically (alkali base) dissolved (20–40 wt%) in hexylene glycol (HG) and distilled directly from the reaction mixture as the spirosiloxane [(C6H14O2)2Si, SP] at 200 °C. SP can be lithiated using controlled amounts of LiNH2 to produce LixSiON oligomers/polymers with MWs up to ∼1.5 kDa as characterized by FTIR, MALDI-ToF, multinuclear NMR, TGA-DTA, XRD, XPS, SEM and EDX. XPS analyses show that Li contents depend solely on added LiNH2 but found N contents are only ≤1 at%. NH2 likely is removed as NH3 during sample preparation (vacuum/overnight). In contrast, MALDI indicates N contents of ∼5–30 at% N with shorter drying times (vacuum/minutes). 7Li NMR positive chemical shifts suggest that precursor bound Li+ ions dissociate easily, beneficial for electrochemical applications. The 7Li shifts correlate to Li contents as well as Li+ conductivities. 1H, 13C and 29Si NMRs of the Li6SiON precursor show fluxional behavior implying high Li+ mobility. Dense microstructures are observed for Li4SiON and Li6SiON pellets heated to 200 °C/2 h/N2. Impedance studies suggest that ionic conductivities increase with Li content; the Li6SiON precursor offers the highest ambient conductivity of 8.5 × 10−6 S cm−1 after heating to 200 °C/2 h/N2.


 Please wait while we load your content...
Please wait while we load your content...
