Catalytic strategy for conversion of fructose to organic dyes, polymers, and liquid fuels†
Abstract
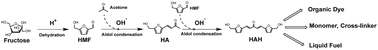
We report a process to produce a versatile platform chemical from biomass-derived fructose for organic dye, polymer, and liquid fuel industries. An aldol-condensed chemical (HAH) is synthesized as a platform chemical from fructose by catalytic reactions in acetone/water solvent with non-noble metal catalysts (e.g., HCl, NaOH). Then, selective reactions (e.g., etherification, reduction, dimerization) of the functional groups, such as enone and hydroxyl groups, in the HAH molecule enable applications in organic dyes and polyether precursors. High yields of target products, such as 5-(hydroxymethyl) furfural (HMF) (85.9% from fructose) and HAH (86.3% from HMF) are achieved by sequential dehydration and aldol-condensation with a simple purification process (>99% HAH purity). The use of non-noble metal catalysts, the high yield of each reaction, and the simple purification of the target product allow for beneficial economics of the process. Techno-economic analysis indicates that the process produces HAH at minimum selling price (MSP) of $1958 per ton. The MSP of HAH product allows the economic viability of applications in organic dye and polyether markets by replacing its counterparts, such as anthraquinone ($3200-$3900 per ton) and bisphenol-A ($1360-$1720 per ton).


 Please wait while we load your content...
Please wait while we load your content...
