Eco-friendly organocatalyst- and reagent-controlled selective construction of diverse and multifunctionalized 2-hydroxybenzophenone frameworks for potent UV-A/B filters by cascade benzannulation†
Abstract
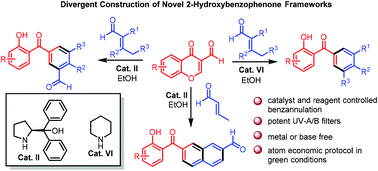
The organocatalyst- and reagent-controlled highly selective synthesis of diversely functionalized novel 2-hydroxybenzophenone frameworks, such as 2-hydroxy-3′-formylbenzophenones, 7-(2′-hydroxybenzoyl)-2-naphthaldehydes, and 2-hydroxybenzophenones, under green conditions, for the development of potent UV-A/B filters is described. The organocatalyzed benzannulation reactions proceed individually via [3 + 3] cycloaddition for the synthesis of 2-hydroxy-3′-formylbenzophenones and [4 + 2] cycloaddition for 2-hydroxybenzophenones. With this methodology, an unprecedented double benzannulation allows one-pot construction of diverse 7-(2′-hydroxybenzoyl)-2-naphthaldehydes via [3 + 3 + 4] cycloaddition. This protocol features a broad substrate scope, high functional-group tolerance, and operational simplicity in an environmentally benign green solvent. The synthesized compounds are successfully utilized for further transformations and well characterized as potent UV-A/B filters.


 Please wait while we load your content...
Please wait while we load your content...