Environmentally benign access to isoindolinones: synthesis, separation and resource recycling†
Abstract
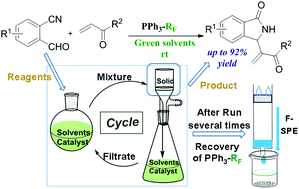
We have developed a green and facile approach for the straightforward installation of isoindolinone skeletons via a tandem reaction of 2-cyanobenzaldehydes and α,β-unsaturated ketones/esters. In the presence of catalytic amounts of the organocatalyst, fluorous phosphine, in green solvents at rt, a variety of isoindolinones were obtained in good to excellent yields without tedious column chromatography. Moreover, both the catalyst and the solvents could be recycled, which greatly reduced the consumption and waste of resources. The simplicity of manipulation, high efficiency of resource utilization and environmentally benign characteristics enable this protocol to have broad applications in the synthesis of isoindolinones, especially those for drug discovery.


 Please wait while we load your content...
Please wait while we load your content...