Design rules for environmental biodegradability of phenylalanine alkyl ester linked ionic liquids†
Abstract
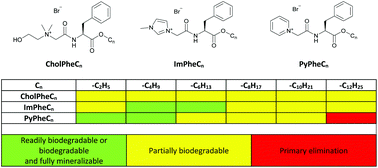
Ionic liquids (ILs) are perceived as a promising group of chemicals within the realm of green chemistry. They are increasingly of interest for open environmental applications. Rules for the design of biodegradable and preferably completely mineralizing ILs after their introduction into the environment are highly desirable. In this study, the impact of the length of the alkyl chain and of the cationic head group on the environmental biodegradability of L-phenylalanine ester (PheCn) derived ILs using the pyridinium (PyPheCn), imidazolium (ImPheCn) and cholinium (CholPheCn) head groups was systematically studied. This included high-resolution mass spectrometry monitoring of formed products of incomplete mineralization. Completely biodegradable ILs were only identified in the PyPheCn series. PyPheC2 and PyPheC4 were fully mineralizable within 42 days with no detectable transformation products (TPs), whereas mineralization of longer chain length PyPheCn ILs required more time. Our results show biodegradation by microorganisms was affected by an alkyl chain length of at least 10 carbon atoms. Biodegradation of the ILs started with biotic ester hydrolysis independent of the alkyl chain length or core scaffold. In contrast, the second step of microbial degradation was an amide bond cleavage which was only shown for ILs with a pyridinium or imidazolium core, even after a test prolongation up to 42 days. Hence, the nature of the cationic head group and the chain length impacted on the mineralization and biodegradability of the ILs. For ILs with an imidazolium core, amide bond cleavage resulted in the formation of recalcitrant 1-carboxymethylimidazolium (ImAc). Whereas for most ILs with a pyridinium core, amide bond cleavage resulted in 1-carboxymethylpyridinium (PyAc) which was mineralizable, although not readily biodegradable according to the test guideline 301D. In the case of cholinium ILs, no further degradation of cholinium phenylalanine amide TP was observed after the hydrolysis of the ester to the alkyl alcohol. The biodegradability of ILs decreased in the order PyPheCn > ImPheCn > CholPheCn, but a key finding is that ImPhe is degraded to ImAc but not further (cf. favourable PyAc mineralisation). These results can be used to design ILs with the property to be effectively biodegraded and mineralized in the environment.


 Please wait while we load your content...
Please wait while we load your content...