An acid-catalyzed 1,4-addition isocyanide-based multicomponent reaction in neat water†
Abstract
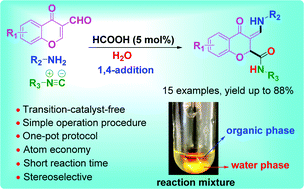
A green 1,4-addition isocyanide-based three-component reaction was developed to synthesize chromanones via a facile, mild, and efficient one-pot protocol without a metal catalyst. The 1,4-addition of isocyanides occurred at the C-2 position of chromones instead of the Schiff base in neat water. We found that the hydrogen bond can dictate the stereo-outcome of olefins, providing an effective method for the diastereomer-selective synthesis of this type of chromanone. The novel green synthetic protocol allowed the quick synthesis of complex chromanones in an environmentally friendly manner.


 Please wait while we load your content...
Please wait while we load your content...