Efficient conversion of CO2 into cyclic carbonates at room temperature catalyzed by Al-salen and imidazolium hydrogen carbonate ionic liquids†
Abstract
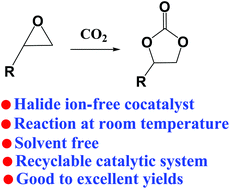
A novel process for the efficient synthesis of cyclic carbonates from CO2 and epoxides at room temperature in the absence of a solvent has been achieved by using Al-salen complexes as catalysts and imidazolium hydrogen carbonate ionic liquids ([CnCmIm][HCO3]) as cocatalysts. As a halide ion-free cocatalyst, [CnCmIm][HCO3] showed higher catalytic reactivity compared to traditional halogen-containing quaternary ammonium salts (such as (nBu)4NBr) and organic bases. The catalytic system can be used for the cycloaddition of a series of substrates with good to excellent yields at room temperature in the absence of a solvent. Besides, the catalytic system can be easily recycled at least four times without significant loss of catalytic activity. A possible mechanism was proposed, in which Al-salen and carbene activate the epoxides and CO2 respectively.


 Please wait while we load your content...
Please wait while we load your content...