Degradation of poly(ethylene terephthalate) catalyzed by metal-free choline-based ionic liquids†
Abstract
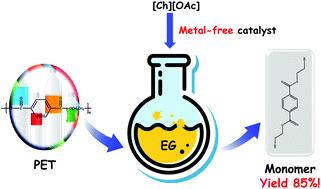
Glycolysis of poly(ethylene terephthalate) (PET) is a prospective way for degradation of PET to its monomer bis(hydroxyethyl) terephthalate (BHET) which can be polymerized again to form new qualified PET materials, and hence provides possibilities for a permanent loop recycling. However, most of the reported glycolysis catalysts are metal-based, leading to high cost and negative environmental impact. In this study, we developed a series of choline-based ionic liquids (ILs) without metals and applied them in the glycolysis of PET as catalysts. Choline acetate ([Ch][OAc]), which is cheaper, more biologically compatible and environmentally friendly in comparison with conventional imidazolium metal-based ILs, can achieve a comparable or even better performance than them. Under optimum conditions (PET (5.0 g), ethylene glycol (EG) (20.0 g), [Ch][OAc] (5 wt%), 180 °C, 4 h, atmospheric pressure), the yield of BHET reached up to 85.2%. Additionally, the reaction kinetics was studied and proved to be the shrinking-core model. The apparent activation energy is 131.31 kJ mol−1, and the pre-exponential factor is 1.21 × 1013 min−1. Finally, based on the experimental results and density functional theory (DFT) calculations, a possible mechanism was proposed. The promotion of the glycolysis reaction is attributed to the activation of EG by the formation of hydrogen bonds between EG and the IL.


 Please wait while we load your content...
Please wait while we load your content...