Synthesis of sulfone-functionalized chroman-4-ones and chromans through visible-light-induced cascade radical cyclization under transition-metal-free conditions†
Abstract
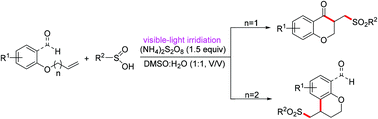
A highly efficient and straightforward approach has been developed for selective intramolecular radical cyclization of arylsulfinic acids with o-(allyloxy)arylaldehydes, o-(homoallyloxy)arylaldehydes and (but-3-en-1-yloxy)benzenes. This photoinduced reaction generated sulfone-functionalized chroman-4-ones and chromans in good to excellent yields under simple and mild conditions without an external photocatalyst.


 Please wait while we load your content...
Please wait while we load your content...