General sulfone construction via sulfur dioxide surrogate control†
Abstract
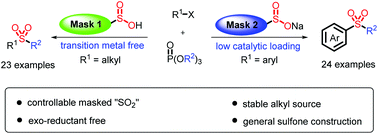
A highly efficient one-step synthesis of alkyl–alkyl and aryl–alkyl sulfones with a facile combination of halides, sulfur dioxide surrogates and phosphate esters is described. When thiourea dioxide was employed as a reductive sulfur dioxide surrogate, alkyl–alkyl sulfones were obtained under transition metal free conditions. Aryl–alkyl sulfones were obtained with an extremely low catalytic loading (0.2 mol%) via altering the mask of sulfur dioxide surrogates to sodium dithionite. A phosphate ester was employed as a stable and readily available alkyl source. Notably, this protocol has been applied to the late-stage modification of natural products and bioactive molecules.


 Please wait while we load your content...
Please wait while we load your content...