The salt-free nickel-catalysed α-allylation reaction of ketones with allyl alcohol and diallylether†
Abstract
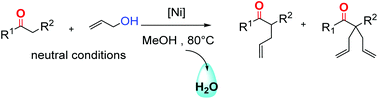
The nickel-catalysed α-allylation of ketones with allyl alcohol and diallylether has been performed under neutral conditions. As no base is involved, the products are synthesized without salts as side products. The dppf/Ni(cod)2 catalytic system in MeOH at 80 °C has been shown as the most effective reaction system to afford tetrasubstituted derivatives from various cyclic and acyclic ketones with one or two mobile protons. This process combined with a metathesis step yields spirocyclic compounds according to a salt free synthetic sequence.


 Please wait while we load your content...
Please wait while we load your content...