Ni-Catalyzed reductive amination of phenols with ammonia or amines into cyclohexylamines†
Abstract
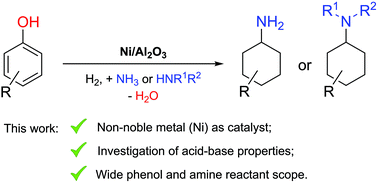
Phenol and its derivatives, which naturally occur in lignocellulose, can be considered as a renewable feedstock not only for aromatic, but also for alicyclic compounds, such as primary and N-substituted cyclohexylamines. So far, the latter are mostly produced from non-renewable starting materials like benzene via problematic nitration/reduction or cross-coupling routes. Herein, an efficient reductive amination of phenol with ammonia or amines is demonstrated, for the first time without the need for rare and expensive noble metals and without using any additives. Various supported Ni catalysts were screened and we elucidated the influence of the key parameters, including the acid–base properties of the supporting material. Acquired knowledge was then applied to different phenol–ammonia/amine combinations, resulting in the synthesis of various primary, secondary and tertiary cyclohexylamines in fair to very high yields.
- This article is part of the themed collection: International Symposium on Green Chemistry 2019


 Please wait while we load your content...
Please wait while we load your content...