Electrochemical TEMPO-catalyzed multicomponent C(sp3)–H α-carbamoylation of free cyclic secondary amines†
Abstract
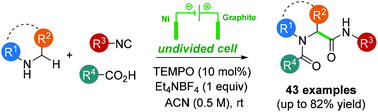
We report here an original electrosynthetic method allowing the straightforward C(sp3)–H α-carbamoylation of free cyclic secondary amines. Based on a TEMPO-catalyzed indirect anodic oxidation and a multicomponent coupling, a wide variety of N-acyl α-carboxamides have been obtained under remarkably mild and sustainable reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...