Transition-metal-free decarboxylative halogenation of 2-picolinic acids with dihalomethane under oxygen conditions†
Abstract
A convenient and efficient method for the synthesis of 2-halogen-substituted pyridines is described. The decarboxylative halogenation of 2-picolinic acids with dihalomethane proceeded smoothly via N-chlorocarbene intermediates to afford 2-halogen-substituted pyridines in satisfactory to excellent yields under transition-metal-free conditions. This new type of decarboxylative halogenation is operationally simple and exhibits high functional-group tolerance.
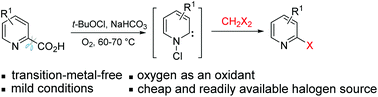


 Please wait while we load your content...
Please wait while we load your content...