The highly efficient air oxidation of aryl and alkyl boronic acids by a microwave-assisted protocol under transition metal-free conditions†
Abstract
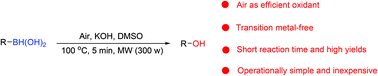
Molecular oxygen is the most important green-oxidant due to its excellent properties. However, the effective utilization of molecular oxygen remains a major challenge in modern chemistry. Herein, we report the development a rapid, green and efficient microwave-assisted protocol for the air oxidation of boronic acids to phenols and alcohols under transition metal-free conditions. In the presence of KOH and DMSO, high yields of the expected phenols and alcohol were obtained with microwave-assistance, and a variety of functional groups were tolerated in this procedure. Notably, this transition metal-free method represents a breakthrough in both organic synthesis and green chemistry for the oxidative hydroxylation of boronic acids to phenols and alcohols.


 Please wait while we load your content...
Please wait while we load your content...