Converting H+ from coordinated water into H− enables super facile synthesis of LiBH4†
Abstract
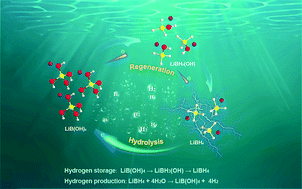
LiBH4 has excellent application potential as an off- or on-board hydrogen carrier for its unparalleled hydrogen capacity (18.5 wt%). However, the difficulty in recycling “one-pass” LiBH4 and the resulting high cost hamper its large-scale applications. Here, we report a facile and low-cost method for LiBH4 regeneration by ball milling its hydrolysis by-product (LiBO2·2H2O) and Mg under ambient conditions, where expensive H− stored in LiBH4 is entirely converted from cheap H+ in coordinated water. This scenario is reported for the first time without hydrides employed as hydrogen sources for the reduction process. Moreover, the yields for LiBH4 synthesized by this method may reach 40%. The cost of LiBH4 regeneration exhibits a 5-fold reduction over a previous study using NaBH4 as a raw material. Significantly, the as-purified product displays even better physicochemical properties than commercial LiBH4, for example, superior hydrolysis kinetics. Overall, a one-step approach, combining hydrogen production and storage in a closed cycle, is proposed in this work. Furthermore, the formation mechanism of LiBH4 is illustrated and the intermediate product, LiBH3(OH), is successfully observed for the first time during the regeneration course.


 Please wait while we load your content...
Please wait while we load your content...