Synthesis of biomass-derived feedstocks for the polymers and fuels industries from 5-(hydroxymethyl)furfural (HMF) and acetone†
Abstract
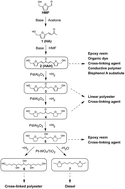
We show that 5-(hydroxymethyl) furfural (HMF) and acetone can be selectively converted into a range of biomass-derived high molecular weight chemicals in high yield (>90%). HMF was condensed with acetone and purified to produce HMF-Acetone-HMF dimer (HAH). We then selectively synthesized various compounds from HAH by the sequential catalytic reaction pathway of hydrogenation, ring-opening, and hydrodeoxygenation. These synthesized HMF-derived chemicals were characterized by 1D and 2D NMR spectra and high resolution mass spectrometry (HRMS). The HMF-derived molecules described in this paper can be feedstocks for the polymer, pigment, and petroleum industries.


 Please wait while we load your content...
Please wait while we load your content...
