Metal-free visible-light induced cyclization/substitution cascade reaction of alkyne-tethered cyclohexadienones and diselenides: access to 5-hydroxy-3-selenyl-4a,8a-dihydro-2H-chromen-6(5H)-ones†
Abstract
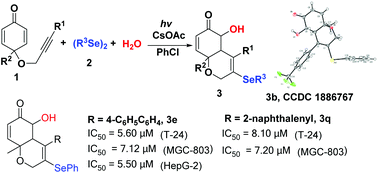
A simple and efficient Se-radical triggered cyclization/substitution cascade reaction of alkyne-tethered cyclohexadienones to afford 5-hydroxy-3-selenyl-4a,8a-dihydro-2H-chromen-6(5H)-ones has been developed. This transformation via the 3,5-diselenyl-4a,8a-dihydro-2H-chromen-6(5H)-one intermediate followed by hydrolysis in the presence of CsOAc affords the desired product 3. The resulting products were tested for their in vitro anticancer activity using MTT assay, and compounds 3e and 3q showed potent cancer cell-growth inhibition activities.


 Please wait while we load your content...
Please wait while we load your content...